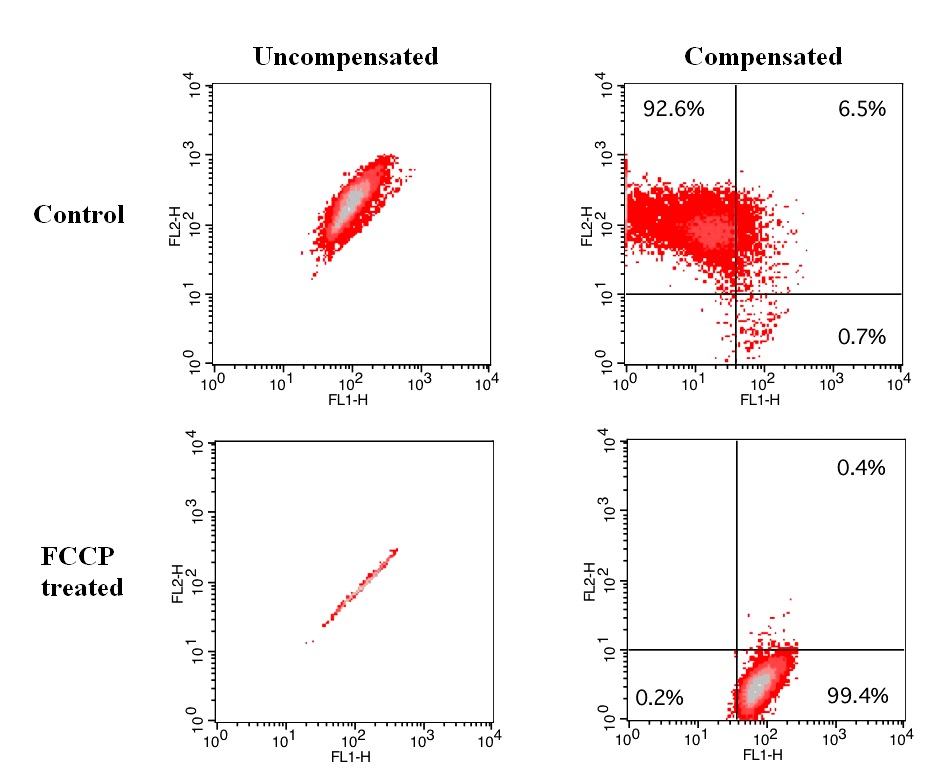
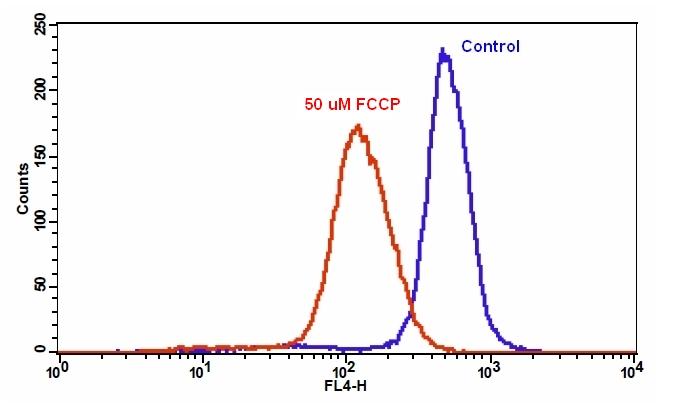
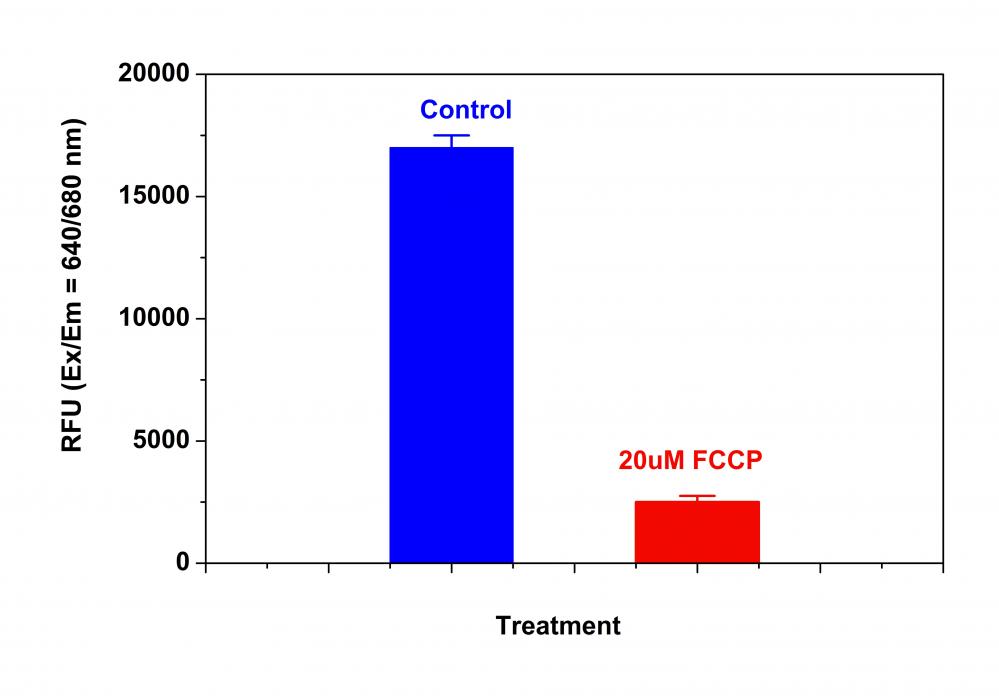
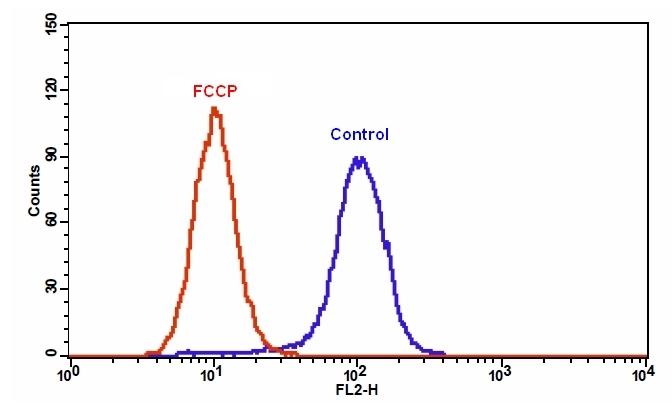
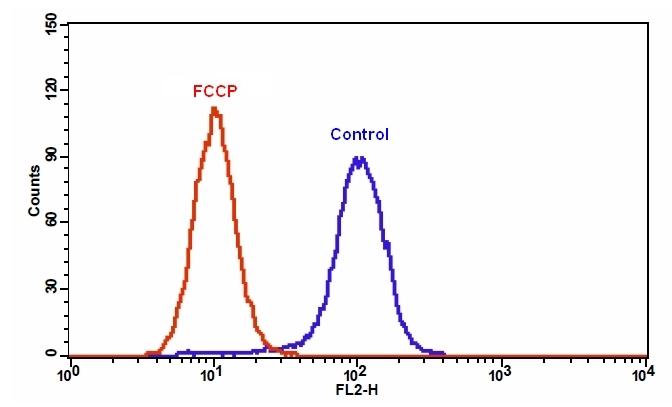
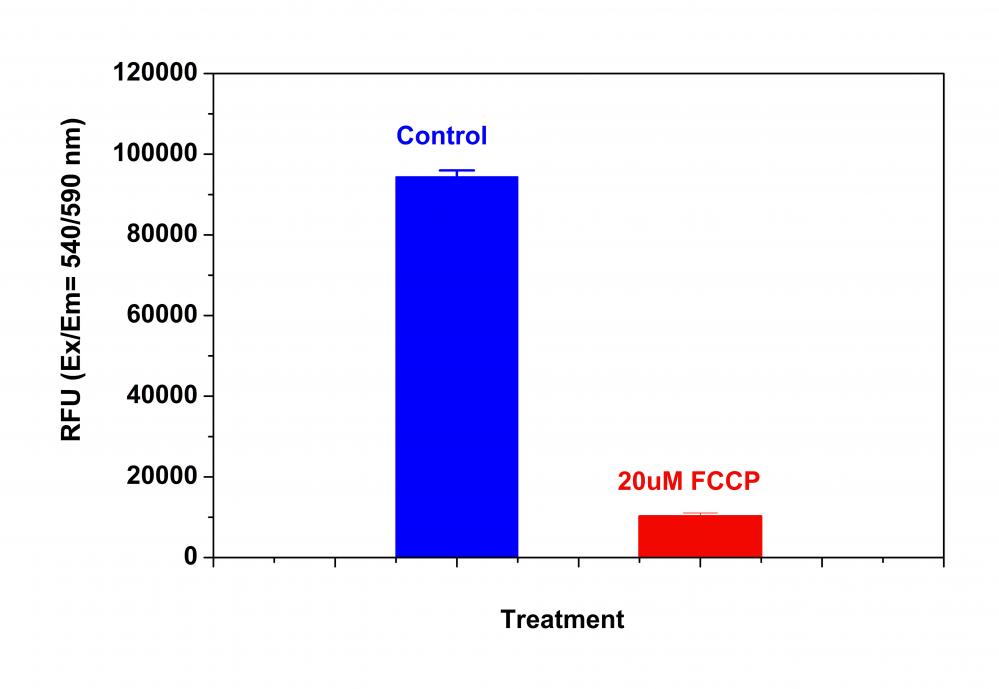
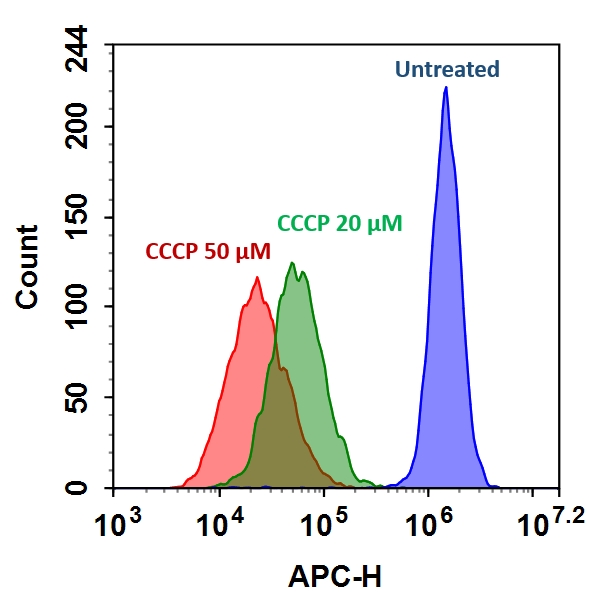
样品实验方案
简要概述
1.用测试化合物制备细胞(100 µL /孔/ 96孔板或25 µL /孔/ 384孔板)
2.加入等量的Apopxin™Violet 450工作溶液
3.在室温下孵育1小时
4.观察Ex / Em = 405/450 nm(截止= 420 nm)的荧光强度(底部读取模式)或使用装有紫罗兰色滤光片的荧光显微镜
溶液配制
工作溶液配制
将10μL100X Apopxin™紫罗兰色450(组分A)添加到1 mL的测定缓冲液(组分B)中,并充分混合以制成Apopxin™紫罗兰色450工作溶液。
操作步骤
1.通过向PBS或所需的缓冲液中加入10X /孔(96孔板)或2.5 µL /孔(384孔板)的10X测试化合物储备溶液来处理细胞。对于空白孔(不含细胞的培养基),添加相同量的化合物缓冲液。
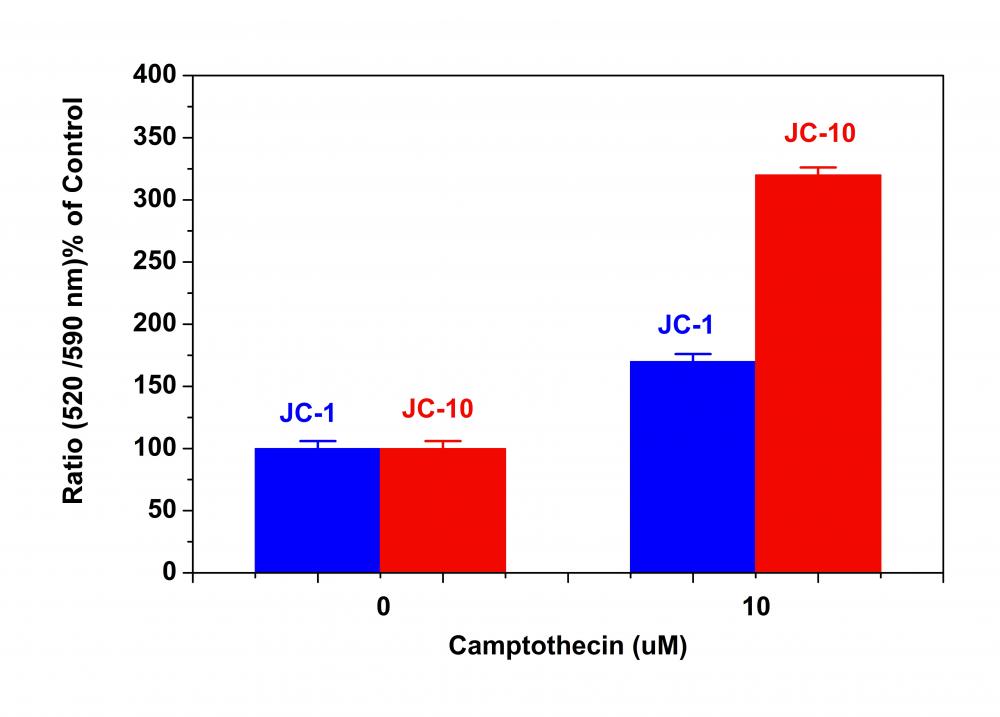
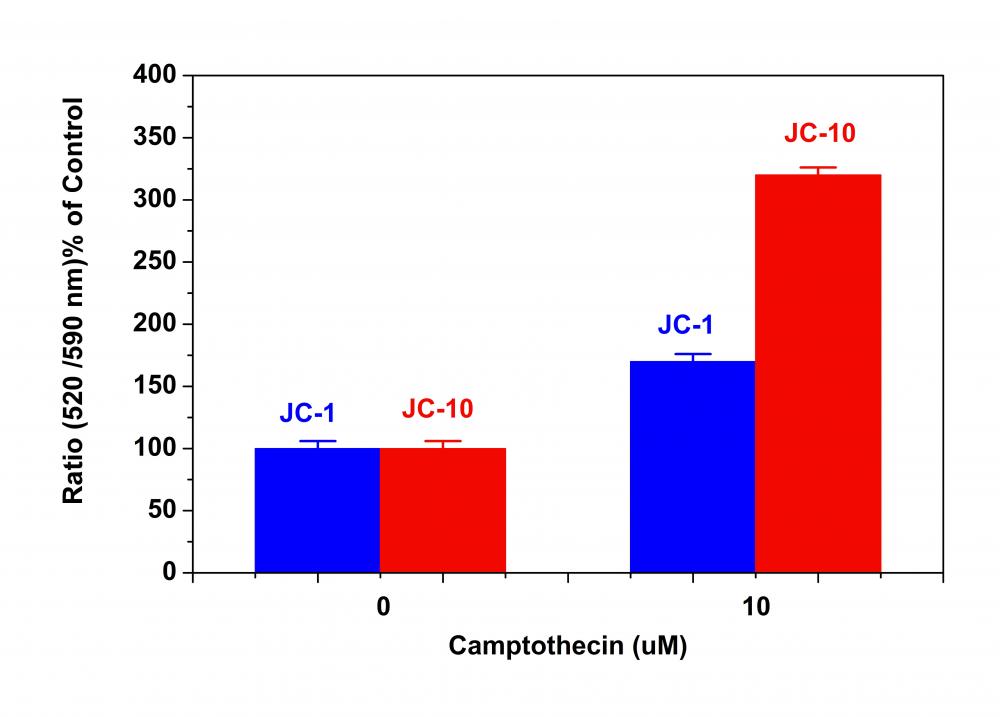
2.将细胞板在5%CO2、37°C的培养箱中孵育所需的时间(对于用星形孢菌素处理的Jurkat细胞需要4-6小时)以诱导凋亡。注意:由于在405 nm激发时,从380至490 nm的宽发射光谱,某些化合物(例如喜树碱)可能会产生假阳性反应。
3.在每个孔中添加100 µL /孔(96孔板)或25 µL /孔(384孔板)的Apopxin™Violet 450工作溶液。
4.在避光条件下,于室温下孵育细胞板至少1小时。
5.以800 rpm的速度离心细胞板(特别是非粘附细胞)2分钟(制动)。
6.使用荧光酶标仪(底部读取模式)在Ex / Em = 405/450 nm(截止= 420 nm)或使用带有紫色滤光片的荧光显微镜对图像池进行荧光强度监测。
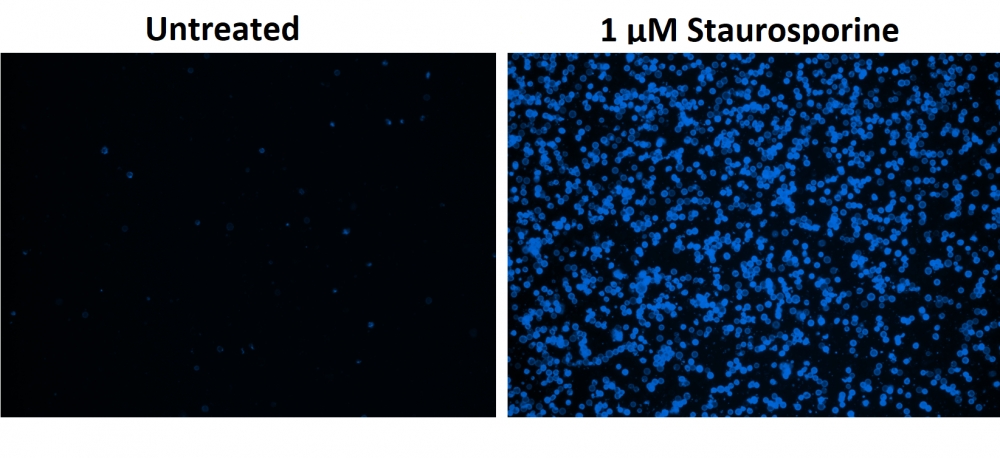
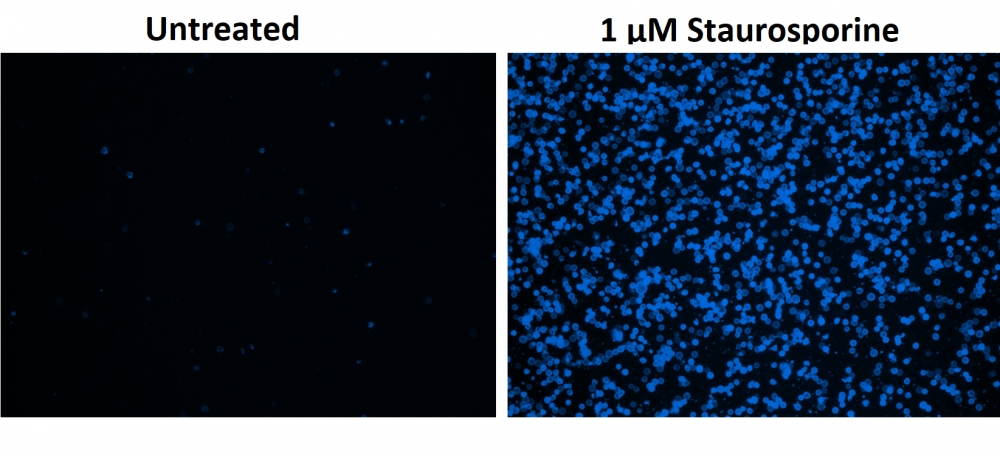
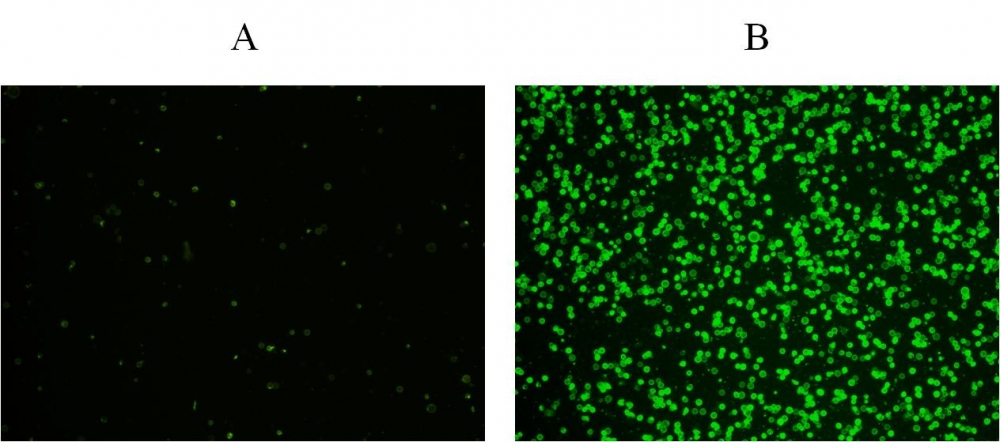
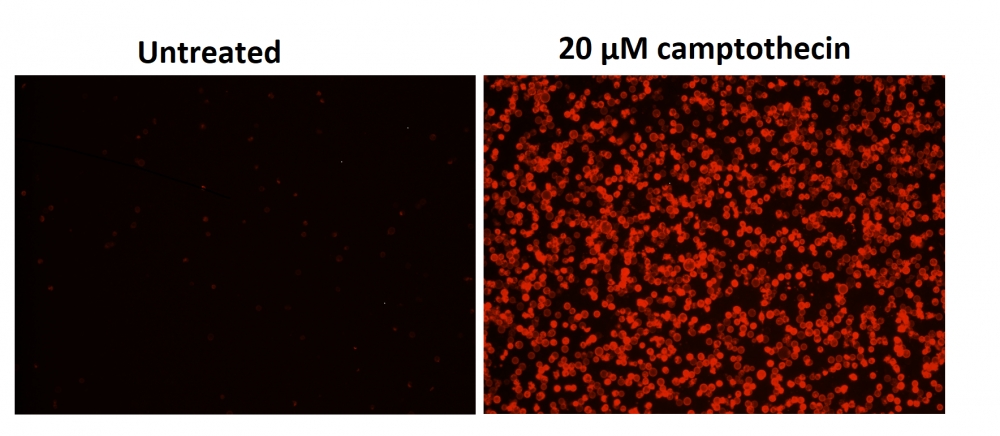
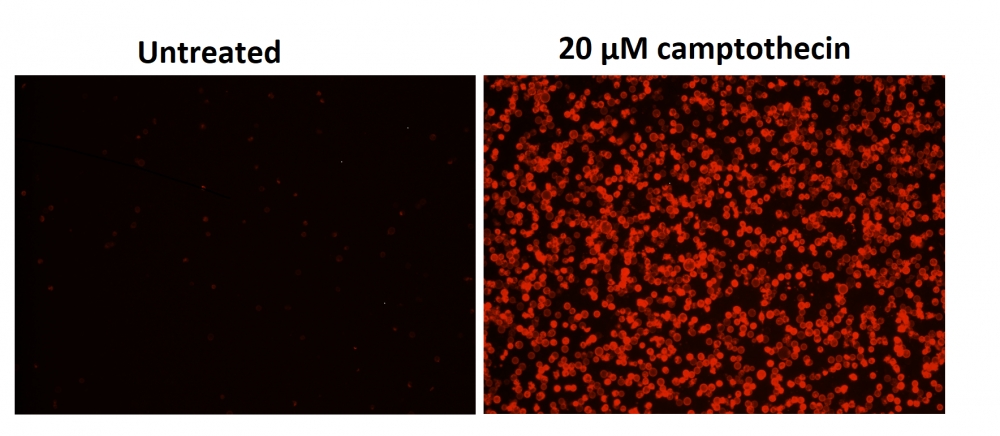
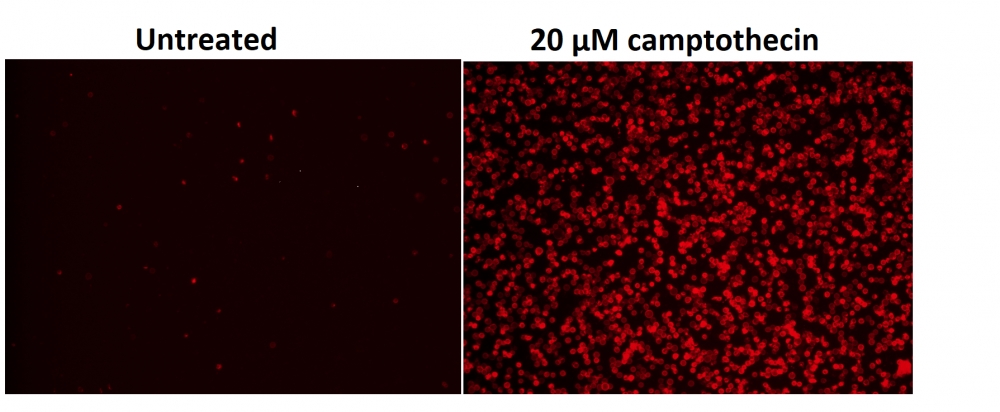
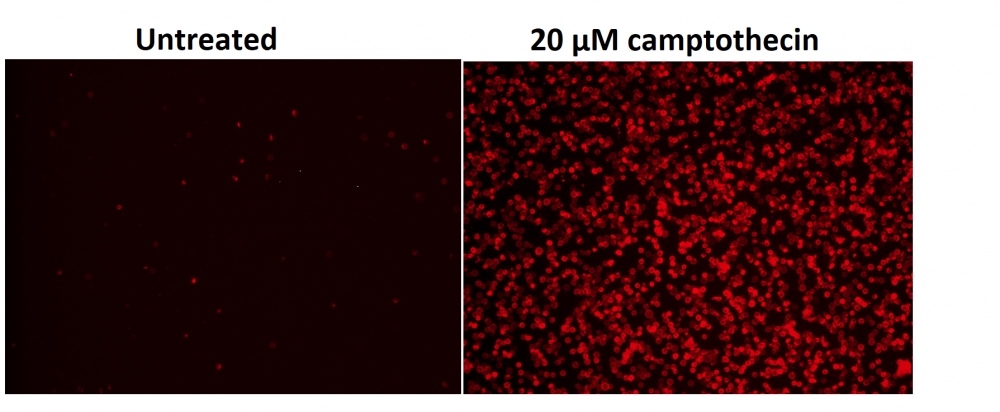
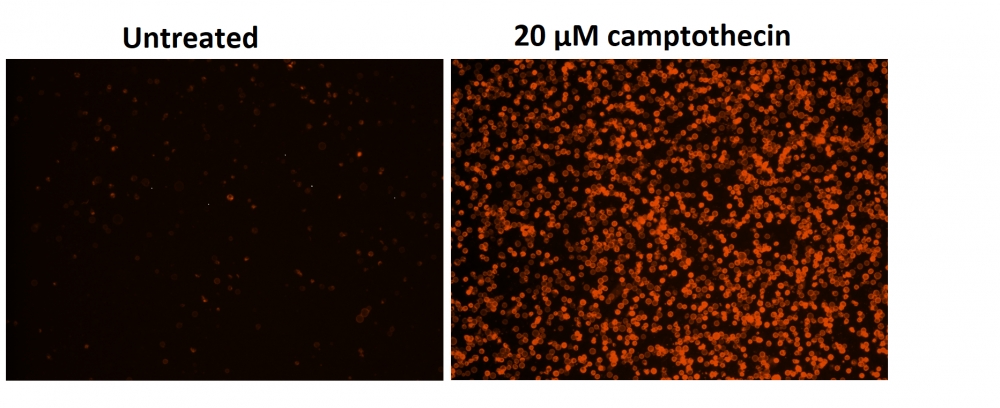
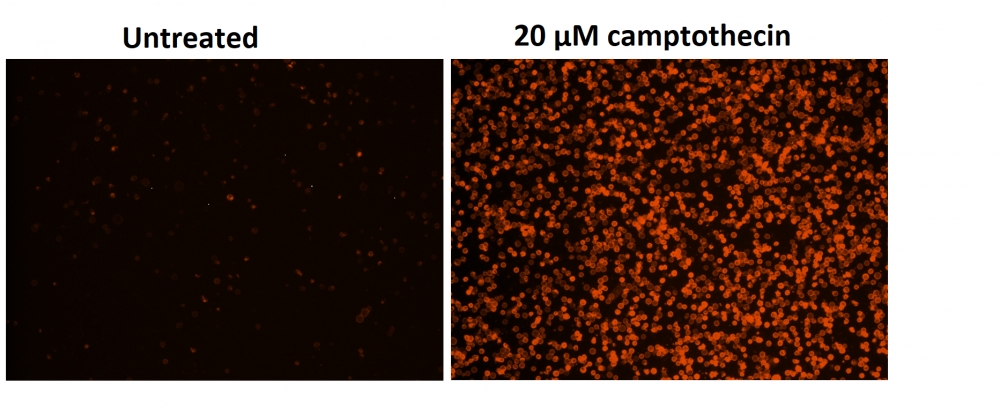
图1.用Apopxin™Violet 450偶联物染色的HeLa细胞的荧光图像。 Jurkat细胞在37℃下不使用(左)或用1μM星形孢菌素(右)处理4小时。 使用带有紫色滤光片组的显微镜(激发= 405nm)测量荧光强度。
参考文献
Physiological effects of the herbicide glyphosate on the cyanobacterium Microcystis aeruginosa
Authors: Wu, Liang and Qiu, Zhihao and Zhou, Ya and Du, Yuping and Liu, Chaonan and Ye, Jing and Hu, Xiaojun
Journal: Aquatic Toxicology (2016): 72–79
Tumor-selective mitochondrial network collapse induced by atmospheric gas plasma-activated medium.
Authors: Saito, Kosuke and Asai, Tomohiko and Fujiwara, Kyoko and Sahara, Junki and Koguchi, Haruhisa and Fukuda, Noboru and Suzuki-Karasaki, Miki and Soma, Masayoshi and Suzuki-Karasaki, Yoshihiro
Journal: Oncotarget (2016)
Inhibition of malignant phenotypes of human osteosarcoma cells by a gene silencer, a pyrrole–imidazole polyamide, which targets an E-box motif
Authors: Taniguchi, Masashi and Fujiwara, Kyoko and Nakai, Yuji and Ozaki, Toshinori and Koshikawa, Nobuko and Toshio, Kojima and Kataba, Motoaki and Oguni, Asako and Matsuda, Hiroyuki and Yoshida, Yukihiro and others
Journal: FEBS open bio (2014): 328–334
Detection of apoptosis based on the interaction between annexin V and phosphatidylserine
Authors: Liu T, Zhu W, Yang X, Chen L, Yang R, Hua Z, Li G.
Journal: Anal Chem (2009): 2410
Evaluation of cell surface expression of phosphatidylserine in ovarian carcinoma effusions using the annexin-V/7-AAD assay: clinical relevance and comparison with other apoptosis parameters
Authors: Dong HP, Holth A, Kleinberg L, Ruud MG, Elstr and MB, Trope CG, Davidson B, Risberg B.
Journal: Am J Clin Pathol (2009): 756
Mobilization of lysosomal calcium regulates the externalization of phosphatidylserine during apoptosis
Authors: Mirnikjoo B, Balasubramanian K, Schroit AJ.
Journal: J Biol Chem (2009): 6918
Peptidic targeting of phosphatidylserine for the MRI detection of apoptosis in atherosclerotic plaques
Authors: Burtea C, Laurent S, Lancelot E, Ballet S, Murariu O, Rousseaux O, Port M, V and er Elst L, Corot C, Muller RN.
Journal: Mol Pharm (2009): 1903
Suicidal membrane repair regulates phosphatidylserine externalization during apoptosis
Authors: Mirnikjoo B, Balasubramanian K, Schroit AJ.
Journal: J Biol Chem (2009): 22512
Trivalent methylated arsenical-induced phosphatidylserine exposure and apoptosis in platelets may lead to increased thrombus formation
Authors: Bae ON, Lim KM, Noh JY, Chung SM, Kim SH, Chung JH.
Journal: Toxicol Appl Pharmacol (2009): 144
Discovery of a phosphatidylserine-recognizing peptide and its utility in molecular imaging of tumour apoptosis
Authors: Thapa N, Kim S, So IS, Lee BH, Kwon IC, Choi K, Kim IS.
Journal: J Cell Mol Med (2008): 1649