样品实验方案
简要概述
(适用于荧光显微镜、荧光酶标仪)
1.在生长培养基中准备细胞
2.用测试化合物处理细胞以诱导ROS
3.添加ROS Brite 570工作溶液(对于96孔板为100 µL /孔,对于384孔板为25 µL /孔)
4.将细胞在37°C下染色30-60分钟
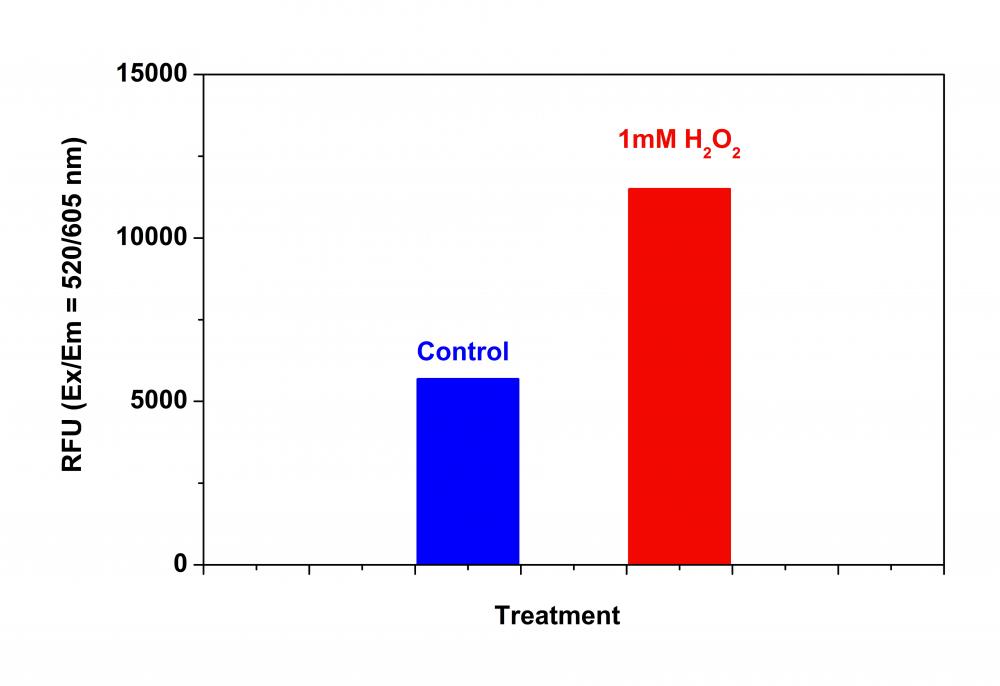
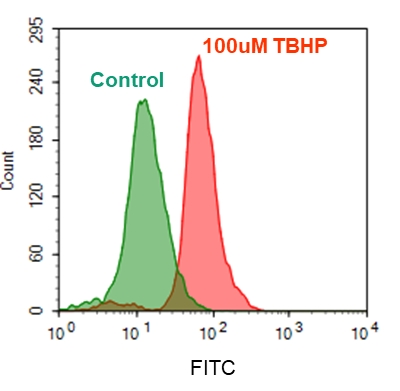
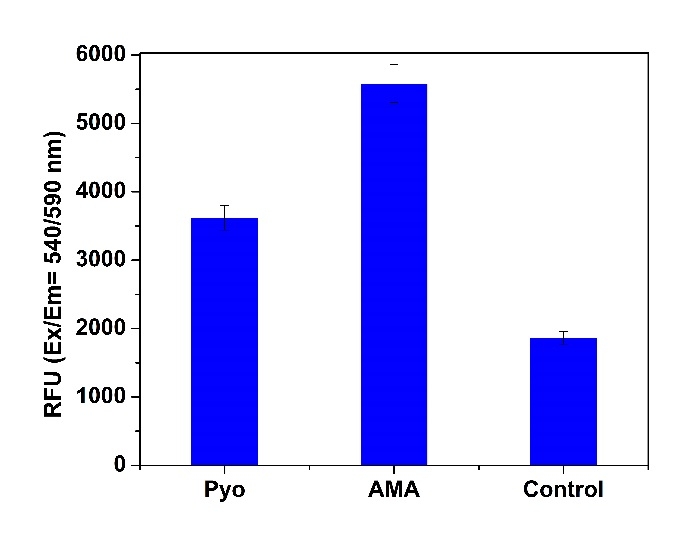
5.在Ex / Em = 540/570 nm(截止= 550 nm)或配备TRITC滤光片的荧光显微镜下检测荧光的增加(底部读取模式)
(流式细胞仪)
1.在生长培养基中准备细胞
2.用测试化合物处理细胞以诱导ROS
3.将ROS Brite 570与细胞一起孵育30-60分钟
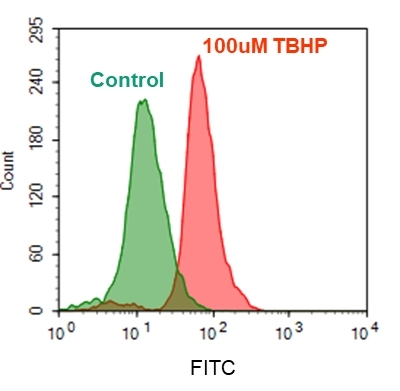
4.使用带有FL2通道的流式细胞仪检测荧光强度
溶液配制
储备溶液配制
1. ROS Brite 570储备溶液(500X):将40 µL DMSO(组分C)添加到ROS Brite 570(组分A)小瓶中,并充分混合以制成500X ROS Brite 570储备液,避光。 注意:20 µL 500X ROS Brite 570储备溶液足以用于1个板。 对于流式细胞仪,为方便起见,可以将5倍ROS Brite 570储备液稀释5倍至DMSO中的100倍。 为了存放,请将管子紧紧密封。
工作溶液配制
将20 µL的500X ROS Brite 570储备溶液添加到10 mL的测定缓冲液(组分B)中,并充分混合以制成ROS Brite 570工作溶液。 注意:此ROS Brite 570工作溶液在室温下至少可稳定2小时。
实验步骤
1.针对荧光显微镜和荧光酶标仪:
1.1在所需的缓冲液(例如PBS或HHBS)中,用10 µL 10X测试化合物(96孔板)或5 µL 5X测试化合物(384孔板)处理细胞。 对于对照孔(未处理的细胞),添加相应量的化合物缓冲液。
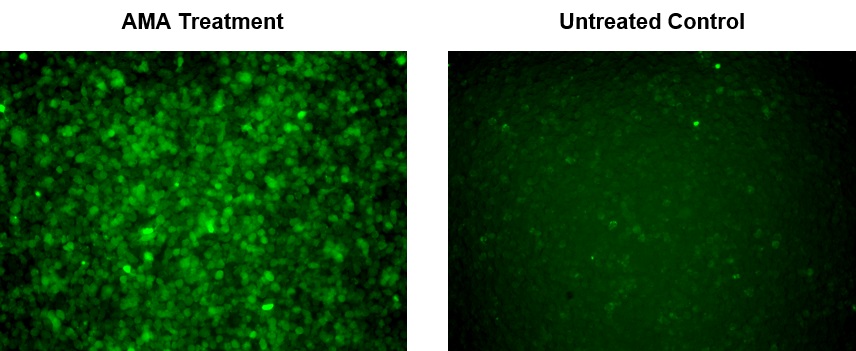
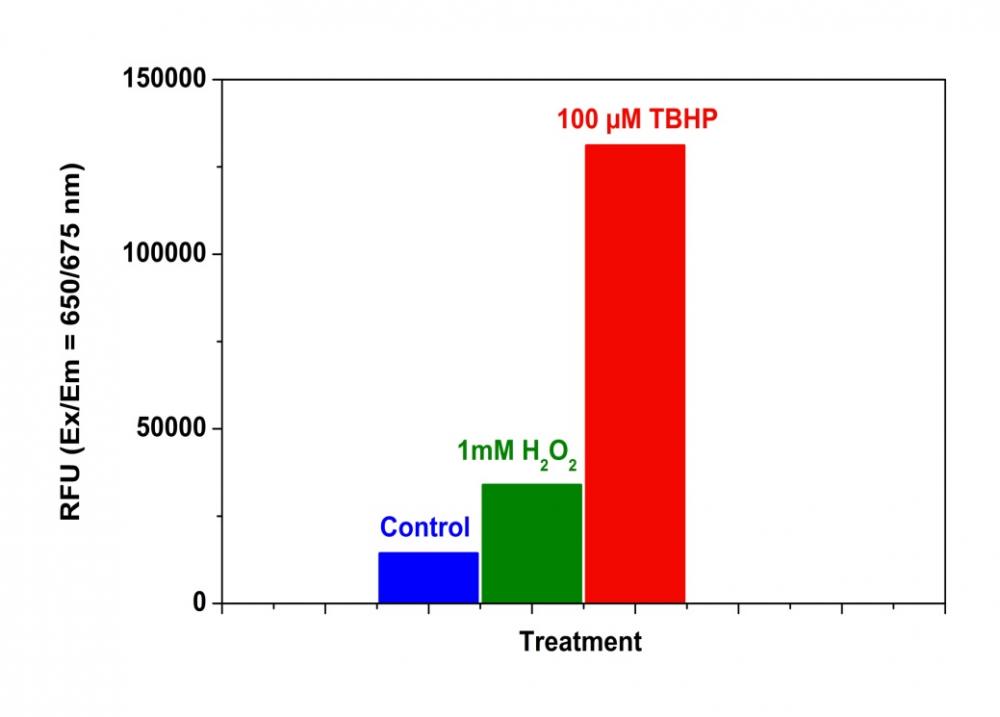
1.2要诱导ROS,请在室温下或在5%CO2、37°C的培养箱中孵育细胞板(例如:用100 µM氢过氧化叔丁基(TBHP)处理Hela细胞30分钟)。
1.3将100 µL /孔(96孔板)或25 µL /孔(384孔板)的ROS Brite 570工作溶液添加到细胞板中。
1.4将细胞在5%CO2、37°C的培养箱中孵育30分钟至60分钟。
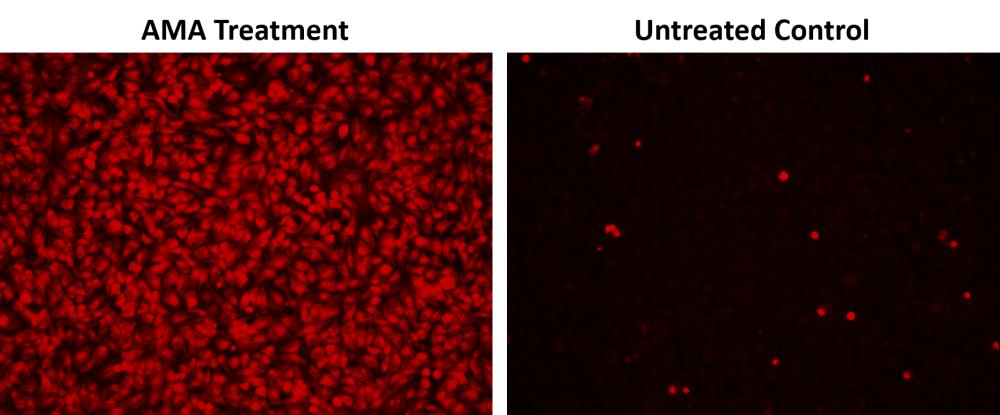
1.5使用荧光酶标仪(Ext / Em = 540/570 nm(截止= 550nm))检测荧光的增加,或使用带有TRITC滤光片组的荧光显微镜检测细胞。
2.针对流式细胞仪:
2.1准备从5×105到1×106细胞/ mL的密度的细胞。 注意:应根据个体情况评估每种细胞系,以确定诱导凋亡的细胞密度。
2.2在所需的缓冲液(例如PBS或HHBS)中用测试化合物处理细胞。 对于对照孔(未处理的细胞),添加相应量的化合物缓冲液。
2.3要诱导ROS,请在室温下或在5%CO2、37°C的培养箱中孵育细胞板至少30分钟(如对于用100 µM氢过氧化叔丁基(TBHP)处理的Hela细胞,则需30分钟) )。
2.4向细胞培养基中加入1 µL / mL的500X ROS Brite 570储备液细胞或5 µL / mL的100X ROS Brite 570储备液细胞。
注:1µL/mL 表示每毫升细胞培养基中添加的ROS Brite 570储备液体积为1微升。这个浓度是相对于细胞培养基的总体积而言的,因此也可以说是在每升细胞培养基中添加1毫升ROS Brite 570储备液。这种浓度通常用于细胞实验中,以使得实验物质与细胞有良好的相互作用,同时不会对细胞造成太大的毒性影响。该浓度仅为初学者进行指导,请根据自己的实际情况,调整浓度,优化自己的实验。
2.5将细胞在5%CO2、37°C的培养箱中孵育30至60分钟。
2.6使用带有FL2通道的流式细胞仪检测荧光强度。
参考文献
Anti-proliferation effect of blue light-emitting diodes against antibiotic-resistant Helicobacter pylori
Authors: Ma, Jianwei and Hiratsuka, Takahiro and Etoh, Tsuyoshi and Akada, Junko and Fujishima, Hajime and Shiraishi, Norio and Yamaoka, Yoshio and Inomata, Masafumi
Journal: Journal of Gastroenterology and Hepatology (2017)
Notoginsenoside R1 attenuates high glucose-induced endothelial damage in rat retinal capillary endothelial cells by modulating the intracellular redox state
Authors: Fan, Chunlan and Qiao, Yuan and Tang, Minke
Journal: Drug Design, Development and Therapy (2017): 3343
Good hydration and cell-biological performances of superparamagnetic calcium phosphate cement with concentration-dependent osteogenesis and angiogenesis induced by ferric iron
Authors: Zhang, J and Shi, HS and Liu, JQ and Yu, T and Shen, ZH and Ye, JD
Journal: Journal of Materials Chemistry B (2015): 8782–8795
Topiramate Protects Pericytes from Glucotoxicity: Role for Mitochondrial CA VA in Cerebromicrovascular Disease in Diabetes
Authors: Patrick, Ping and Price, Tulin O and Diogo, Ana L and Sheibani, Nader and Banks, William A and Shah, Gul N
Journal: Journal of endocrinology and diabetes (2015)
Down-regulated peroxisome proliferator-activated receptor γ (PPARγ) in lung epithelial cells promotes a PPARγ agonist-reversible proinflammatory phenotype in chronic obstructive pulmonary disease (COPD)
Authors: Lakshmi, Sowmya P and Reddy, Aravind T and Zhang, Yingze and Sciurba, Frank C and Mallampalli, Rama K and Duncan, Steven R and Reddy, Raju C
Journal: Journal of Biological Chemistry (2014): 6383–6393
Superoxide dismutase as a target of clioquinol-induced neurotoxicity
Authors: Kawamura, Kazuyuki and Kuroda, Yukiko and Sogo, Masako and Fujimoto, Miki and Inui, Toshio and Mitsui, Takao
Journal: Biochemical and biophysical research communications (2014): 181–185
Xanthine oxidase inhibition by febuxostat attenuates experimental atherosclerosis in mice
Authors: Nomura, Johji and Busso, Nathalie and Ives, Annette and Matsui, Chieko and Tsujimoto, Syunsuke and Shirakura, Takashi and Tamura, Mizuho and Kobayashi, Tsunefumi and So, Alex and er and Yamanaka, Yoshihiro
Journal: Scientific reports (2014): 4554
High glucose-induced mitochondrial respiration and reactive oxygen species in mouse cerebral pericytes is reversed by pharmacological inhibition of mitochondrial carbonic anhydrases: implications for cerebral microvascular disease in diabetes
Authors: Shah, Gul N and Morofuji, Yoichi and Banks, William A and Price, Tulin O
Journal: Biochemical and biophysical research communications (2013): 354–358
Automatic flow injection based methodologies for determination of scavenging capacity against biologically relevant reactive species of oxygen and nitrogen
Authors: Magalhaes LM, Lucio M, Segundo MA, Reis S, Lima JL.
Journal: Talanta (2009): 1219
Diabetes and the impairment of reproductive function: possible role of mitochondria and reactive oxygen species
Authors: Amaral S, Oliveira PJ, Ramalho-Santos J.
Journal: Curr Diabetes Rev (2008): 46